厚生労働科学研究費補助金(難治性疾患克服研究事業) 「Menkes 病・occipital horn 症候群の実態調査、早期診断基準確立、治療法開発に関する研究」 平成23年度 総括・分担研究報告書(page 40/118)[厚生労働科学研究費補助金(難治性疾患克服研究事業) 「Menkes 病・occipital horn 症候群の実態調査、早期診断基準確立、治療法開発に関する研究」 平成23年度 総括・分担研究報告書]
このページは、電子ブック 「厚生労働科学研究費補助金(難治性疾患克服研究事業) 「Menkes 病・occipital horn 症候群の実態調査、早期診断基準確立、治療法開発に関する研究」 平成23年度 総括・分担研究報告書」 内の 40ページ の概要です。
秒後に電子ブックの対象ページへ移動します。
「電子ブックを開く」をクリックすると、今すぐ対象ページへ移動します。
概要:
Inherited Cu Disorders Current Drug Metabolism, 2012, Vol. 13, No. 3 245coppering therapy [82]. The types of seizures can vary, and includegeneralized tonic-clonic (grand mal), simple partial, complex....
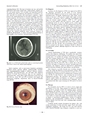
Inherited Cu Disorders Current Drug Metabolism, 2012, Vol. 13, No. 3 245coppering therapy [82]. The types of seizures can vary, and includegeneralized tonic-clonic (grand mal), simple partial, complex partial,and partial seizures with secondary and generalized periodicmyoclonus [82]. Early diagnosis and initiation of treatment is crucial,especially for patients with neurological symptoms. Copperlevels in the cerebrospinal fluid are elevated in patients with neurologicalsymptoms, but decrease to normal ranges following treatment.Thus, copper levels could be a useful marker for monitoringpatients with neurological symptoms [83].“Face of giant pandasign,”tectal plate hyperintensity, central pontine myelinosis (CPMlike),and concurrent changes in basal ganglia, thalamus, and brainstemare observed in MRIs from patients with neurological WD[84,85]. High signal T1 images, similar to those in portal-systemicencephalopathy, are also observed [85]. In addition, loss of cerebralwhite matter has rarely been reported (Fig 12). 31 P- and 1 H-MRSindicate that reduced breakdown and/or increased synthesis ofmembrane phospholipids, as well as increased neuronal damage inbasal ganglia, occur in patients with neurological WD [86].Fig. (12). Loss of left frontal cerebral white matter in a neurological patientwith Wilson’s disease who suffered right hemiplegia.Initial symptoms, such as microscopic hematuria, proteinuria,hemolytic anemia, epistaxis, arthritis, cardiomyopathy, dysrhythmias,hyperpigmentation (similar to Addison’s disease), cataracts,amenorrhea, and hypersalivation, vary and make early diagnosisdifficult [17,87,88]. Kayser-Fleischer rings are also common inneurological WD, which reflect copper deposition in the brain (Fig13). However, about 40% and 20% of patients with hepatic andneurological symptoms, respectively, show no Kayser-Fleischerrings [88-90].Fig. (13). Kayser-Fleischer rings.5.4. DiagnosisGuidelines for the diagnosis of WD were approved in 2008 bythe American Association for the Study of Liver Diseases(AASLD) [89]. Diagnosis is based on low serum copper and ceruloplasminlevels (<20 mg/dL; immunoassay), high copper concentrationsin the liver (>250 ?g/g dry weight), high copper excretionin the urine (>100 ?g/day), and by conducting a penicillamine challengetest (urinary copper excretion >1,600 or 1,057 ?g/day)[89.91]. In some patients with WD, however, serum copper andceruloplasmin levels are not low [88,89]. In fact, serum copperlevels are often high in patients with WD suffering from acute liverfailure due to the release of accumulated copper in hepatocytes.Furthermore, other hepatic diseases, including autoimmune hepatitisand intrahepatic cholestasis, may affect serum copper measurementsand make diagnosis difficult. DNA-based diagnosis (e.g.,high-resolution melting analysis or HRM) has also been reported[92]. However, approximately 17% of patients diagnosed with WDbased on clinical symptoms and biochemical data have no mutationsin the coding regions of ATP7B [69]. Scoring systems for thediagnosis of WD have been proposed in order to account for thedeficiencies of any one test [93]. Although a diagnosis can be madein the vast majority of cases, a small number of patients cannot bediagnosed with the tests described above [94]. Once a patient isdiagnosed with WD, all first- and second-degree relatives shouldalso be screened for the disease. Treatment should be offered topresymptomatic patients, although diagnosis in some cases can bechallenging.5.5. ScreeningClinical manifestations of WD show considerable variation,making early diagnosis challenging. The median time interval betweenpresentation of initial symptoms and diagnosis is 18 months(ranging from 1-72 months) for patients with neurological symptomsand 6 months (ranging from 2-108 months) for patients withhepatic symptoms [90]. Despite current strategies, the mean delayfrom presentation of initial symptoms to diagnosis is two years(ranging from 0.08-30 years) [95]. This is mainly due to the lowawareness and index of suspicion by primary care physicians [95].Awareness and diagnosis could be improved by implementingmedical education strategies that target primary care physicians.Early diagnosis is possible through mass screening strategies,which also enable the detection of presymptomatic patients. Holoceruloplasmindetections in newborn blood or in urine of 3-6 yearoldchildren have been proposed as potential mass screening strategies[96-98]. To date, however, mass screening has not yet beenimplemented anywhere. The specificity and sensitivity of thesemethods require further investigation, as well as a cost-benefitanalysis when applied at the population level. Ultimately, innovativemethods that allow mass screening for WD need to be developed.5.6. TherapyThe therapeutic aim for WD is to remove excess copper thataccumulates in the body. When patients are diagnosed with WD,they should be promptly treated with chelating agents, includingpenicillamine and trientine, and/or zinc (Table 3) [89]. Chelatingagents should be taken on an empty stomach because food preventstheir absorption. These agents are usually recommended to be taken1 hour before or 2 hours after meals. The treatment choice dependson hepatic or neurological manifestations, severity of symptoms,pregnancy, and presymptomatic conditions [89,99]. In addition,patients should avoid food and water containing high concentrationsof copper.Treatment should continue throughout the patient’s life, withroutine monitoring of serum and urine copper, blood cell counts,coagulation parameters, and testing for liver and renal function[100]. Kayser-Fleischer rings disappear completely in most pa-